
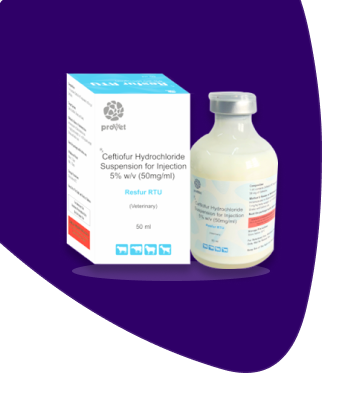
Resfur RTU
For Reproductive & Respiratory Diseases
Ceftiofur Hydrochloride Sterile Suspension

Calject
Milk Fever Prevention & Treatment
Calcium Borogluconate Injection IP (Vet.)

M-ject
Powerful solution to treat Milk Fever complex
Calcium Magnesium Borogluconate Injection IP (Vet.)

Resfur RTU
For Reproductive & Respiratory Diseases
Ceftiofur Hydrochloride Sterile Suspension

Calject
Milk Fever Prevention & Treatment
Calcium Borogluconate Injection IP (Vet.)

M-ject
Powerful solution to treat Milk Fever complex
Calcium Magnesium Borogluconate Injection IP (Vet.)
Our Latest
Product Launches
Our Latest Product Launches

Calject
Milk Fever Prevention & Treatment
Calcium Borogluconate Injection IP (Vet.)

M-ject
Powerful solution to treat Milk Fever complex
Calcium Magnesium Borogluconate Injection IP (Vet.)

Resfur RTU
For Reproductive & Respiratory Diseases
Ceftiofur Hydrochloride Sterile Suspension
Leveraging our Manufacturing and R&D Capabilities
Leaveraging our Manufacturing & R&D Capabilities
We are Alivira Animal Health Limited

Centres








Serving Our Community
We believe in fostering inclusive growth and sustainable development. By identifying key areas for focused intervention, we undertake initiatives that drive meaningful and lasting transformation. Our social programs are designed to support the holistic development of the communities in which we operate.
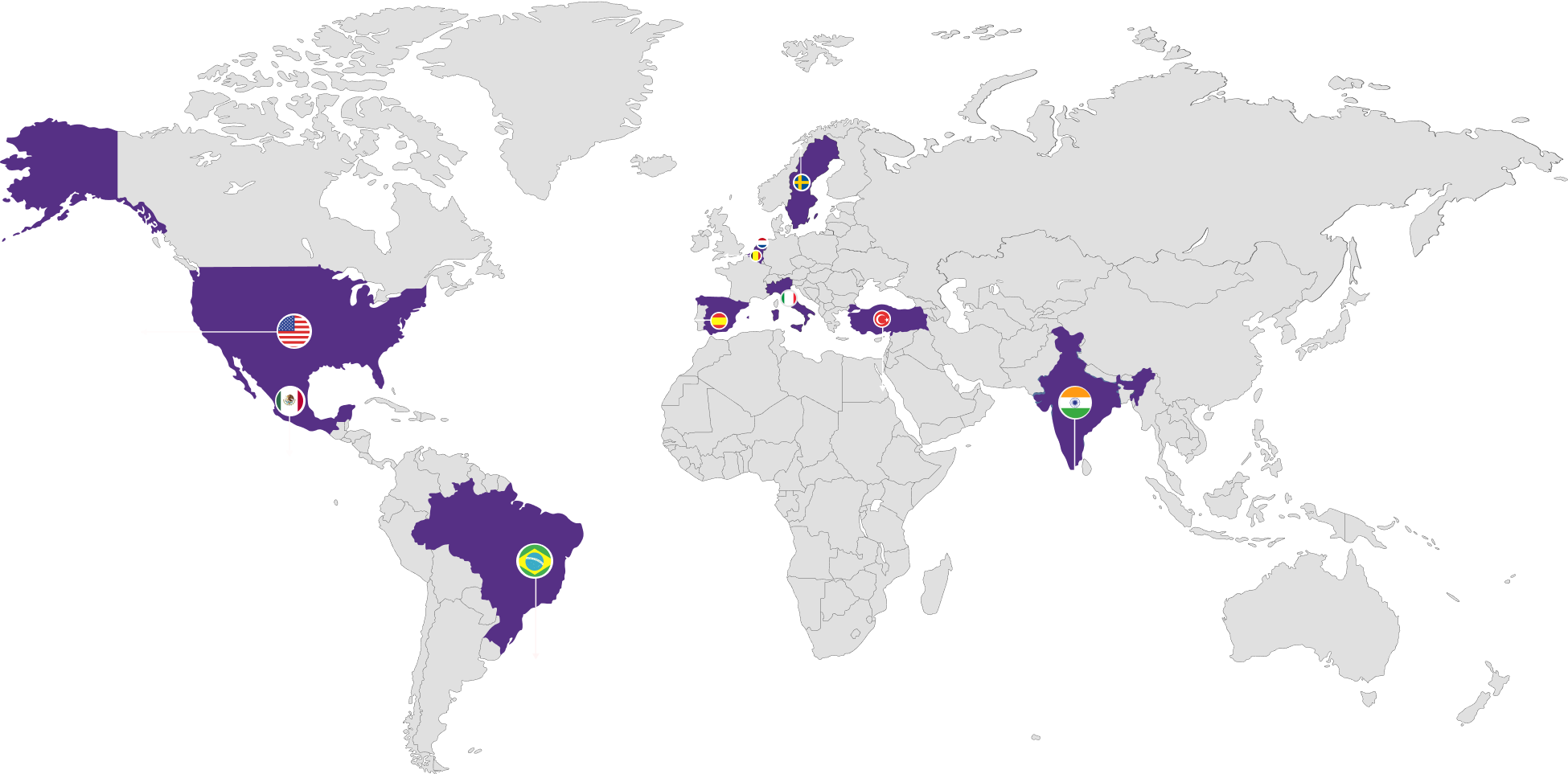
Our Global Reach
Spanning over 5 continents across 100 countries
Location: Ambernath, Vizag, Mahad
Capabilities: Sterile granules, API facility
with reactor capacity of 270 KL with seven
clean rooms, API facility with reactor capacity
of 80KL with two clean rooms
Dosage forms: Oral solutions/suspensions,
powders and premixes
Approvals: cGMP, India, Uganda, Ethiopia,
Kenya, USFDA, WHO-Geneva, COFEPRIS Mexico
Location:
Campinas/SP & Uberlândia/MG
Capabilities:
Dedicated on macrolides,
quinolones, lincosamides, anti-inflammatory
NSAIDs & additives and nutritional
supplements
Dosage forms: Powders, soluble powders and
premixes, oral solutions / suspensions, paste,
tablets and chewable tablets
Approvals: Brazil GMP
Location: Ankara
Capabilities: Beta-lactam block, Hormones
Dosage forms: Sterile suspensions
& Injectable, dry powder, aerosols,
intramammaries, pour-on/spot-on,
oral solutions/ suspensions
Approvals: EUGMP, Turkish GMP
Location: Barcelona
Capabilities: Dedicated beta-lactum powder
block & nutritional
Dosage forms: Oral solutions/ suspensions,
powders and premixes
Approvals: EUGMP
Location: Leuven
Capabilities: Distributors
Sales Unit
Sales Unit
Location: Uppsala
Capabilities: Sales Unit
Location: Ferrara
Capabilities: Sales Unit
Capabilities: Distributors












